Sector
Decarbonization, Green energy, Science & technology
Industry
Automotive, Marine, Aero space, Energy storage, Decarbonization
Technology origin
Europe
Technology status
Available for commercial deployment.
Principal of operation
WHY HYDROGEN FUEL CELL -
Fuel cells can operate at higher efficiencies than combustion engines, and can convert the chemical energy in the fuel to electrical energy with efficiencies of up to 60%.
Fuel cells have lower emissions than combustion engines.
Hydrogen fuel cells emit only water, so there are no carbon dioxide emissions and no air pollutants that create smog and cause health problems at the point of operation.
Also, fuel cells are quiet during operation as they have fewer moving parts.
Hydrogen fuel cells can decarbonize the transport sector.
Fuel cells can be used in a wide range of applications, including transportation, material handling, stationary, portable, and emergency backup power applications.
Fuel cells have several benefits over conventional combustion-based technologies currently used in many power plants and passenger vehicles.
Fuel cells have lower emissions than combustion engines.
Hydrogen fuel cells emit only water, so there are no carbon dioxide emissions and no air pollutants that create smog and cause health problems at the point of operation. Also, fuel cells are quiet during operation as they have fewer moving parts.
Fuel cells can operate at higher efficiencies than combustion engines, and can convert the chemical energy in the fuel to electrical energy with efficiencies of up to 60%.
Fuel cells have lower emissions than combustion engines.
Hydrogen fuel cells emit only water, so there are no carbon dioxide emissions and no air pollutants that create smog and cause health problems at the point of operation.
Also, fuel cells are quiet during operation as they have fewer moving parts.
Hydrogen fuel cells can decarbonize the transport sector.
Fuel cells can be used in a wide range of applications, including transportation, material handling, stationary, portable, and emergency backup power applications.
Fuel cells have several benefits over conventional combustion-based technologies currently used in many power plants and passenger vehicles.
Fuel cells have lower emissions than combustion engines.
Hydrogen fuel cells emit only water, so there are no carbon dioxide emissions and no air pollutants that create smog and cause health problems at the point of operation. Also, fuel cells are quiet during operation as they have fewer moving parts.
Read more
Ratings
100 w to mega watt scale
Scope
Turnkey project
Features
WHAT IS FUEL CELL --
A fuel cell is a device that generates electricity through an electrochemical reaction, not by combustion.
In a fuel cell, hydrogen and oxygen are combined to generate electricity, heat, and water vapours.
Fuel cell systems are a clean, efficient, reliable, and quiet source of power. Fuel cells do not need to be periodically recharged like batteries, but instead continue to produce electricity as long as a fuel source is provided.
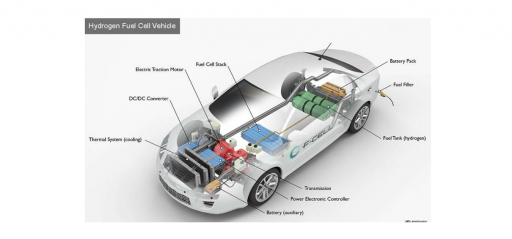
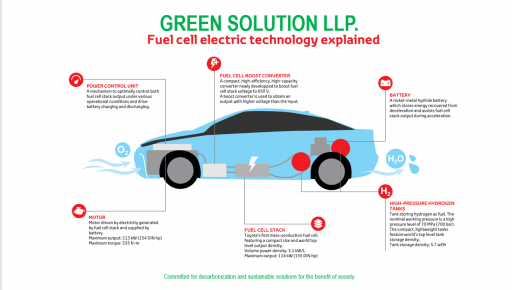
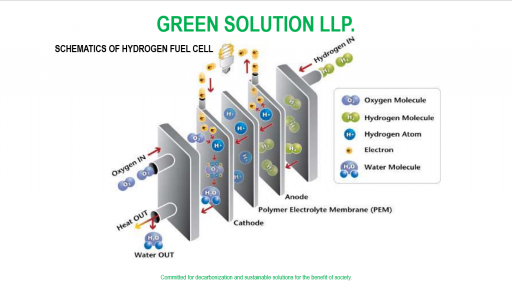
A fuel cell is composed of an anode, cathode, and an electrolyte membrane. A typical fuel cell works by passing hydrogen through the anode of a fuel cell and oxygen through the cathode. At the anode site, a catalyst splits the hydrogen molecules into electrons and protons.
The protons pass through the porous electrolyte membrane, while the electrons are forced through a circuit, generating an electric current and excess heat.
At the cathode, the protons, electrons, and oxygen combine to produce water molecules. As there are no moving parts, fuel cells operate silently and with extremely high reliability.
A fuel cell uses the chemical energy of hydrogen or another fuel to cleanly and efficiently produce electricity.
If hydrogen is the fuel, electricity, water, and heat are the only products.
Fuel cells are unique in terms of the variety of their potential applications; they can provide power for systems as large as a utility power station and as small as a laptop computer.
How Fuel Cells Work --
It is possible to use the reaction between hydrogen and oxygen to generate electricity via fuel cell. In the Apollo space program, such a cell was used and served two distinct purposes: it was used as a source of fuel and a source of drinking water.
A fuel cell can be described as an electrochemical cell which, through an electrochemical reaction, generates electrical energy from the fuel. To maintain the reactions that produce electricity, these cells require a continuous input of fuel and an oxidizing agent (generally oxygen). Thus, before the supply of fuel and oxygen is cut off these cells will continuously produce electricity.
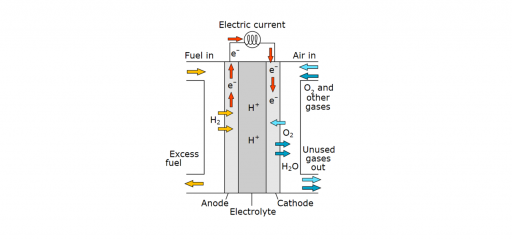
Fuel cells work like batteries, but they do not run down or need recharging. They produce electricity and heat as long as fuel is supplied. A fuel cell consists of two electrodes—a negative electrode (or anode) and a positive electrode (or cathode)—sandwiched around an electrolyte. A fuel, such as hydrogen, is fed to the anode, and air is fed to the cathode. In a polymer electrolyte membrane fuel cell, a catalyst separates hydrogen atoms into protons and electrons, which take different paths to the cathode. The electrons go through an external circuit, creating a flow of electricity. The protons migrate through the electrolyte to the cathode, where they reunite with oxygen and the electrons to produce water and heat
A fuel cell, which consists of a cathode, an anode, and an electrolyte, is similar to electrochemical cells. The electrolyte makes the motion of the protons possible in these cells.
A fuel cell is a device that generates electricity through an electrochemical reaction, not by combustion.
In a fuel cell, hydrogen and oxygen are combined to generate electricity, heat, and water vapours.
Fuel cell systems are a clean, efficient, reliable, and quiet source of power. Fuel cells do not need to be periodically recharged like batteries, but instead continue to produce electricity as long as a fuel source is provided.
A fuel cell is composed of an anode, cathode, and an electrolyte membrane. A typical fuel cell works by passing hydrogen through the anode of a fuel cell and oxygen through the cathode. At the anode site, a catalyst splits the hydrogen molecules into electrons and protons.
The protons pass through the porous electrolyte membrane, while the electrons are forced through a circuit, generating an electric current and excess heat.
At the cathode, the protons, electrons, and oxygen combine to produce water molecules. As there are no moving parts, fuel cells operate silently and with extremely high reliability.
A fuel cell uses the chemical energy of hydrogen or another fuel to cleanly and efficiently produce electricity.
If hydrogen is the fuel, electricity, water, and heat are the only products.
Fuel cells are unique in terms of the variety of their potential applications; they can provide power for systems as large as a utility power station and as small as a laptop computer.
How Fuel Cells Work --
It is possible to use the reaction between hydrogen and oxygen to generate electricity via fuel cell. In the Apollo space program, such a cell was used and served two distinct purposes: it was used as a source of fuel and a source of drinking water.
A fuel cell can be described as an electrochemical cell which, through an electrochemical reaction, generates electrical energy from the fuel. To maintain the reactions that produce electricity, these cells require a continuous input of fuel and an oxidizing agent (generally oxygen). Thus, before the supply of fuel and oxygen is cut off these cells will continuously produce electricity.
Fuel cells work like batteries, but they do not run down or need recharging. They produce electricity and heat as long as fuel is supplied. A fuel cell consists of two electrodes—a negative electrode (or anode) and a positive electrode (or cathode)—sandwiched around an electrolyte. A fuel, such as hydrogen, is fed to the anode, and air is fed to the cathode. In a polymer electrolyte membrane fuel cell, a catalyst separates hydrogen atoms into protons and electrons, which take different paths to the cathode. The electrons go through an external circuit, creating a flow of electricity. The protons migrate through the electrolyte to the cathode, where they reunite with oxygen and the electrons to produce water and heat
A fuel cell, which consists of a cathode, an anode, and an electrolyte, is similar to electrochemical cells. The electrolyte makes the motion of the protons possible in these cells.
Application
Mobile phones, Computer, Automotive use, marine use, Aerospace use, Aeronautical use, Energy generation, Decarbonization, etc.